Analgesia Modalities
Analgesia is defined as the “absence of pain in response to stimulation, which would normally be painful.”1 The type of analgesic prescribed or administered can depend on the source of pain, its severity/intensity and duration, a patient’s medical history, as well as other factors, such as comorbidities, concomitant medications, and their preferences or values.1,2
Non-pharmacologic analgesia
Non-pharmacologic analgesia modalities for pain can be as simple as ice or heat packs, osteopathic manipulative medicine (OMT) or a massage to relieve pain. Other alternative treatments include acupuncture, hypnosis, meditation, tai-chi, and yoga.3,4 Electrical stimulation, biofeedback techniques, psychotherapy, and talk therapy are also methods for patients to cope with and manage pain.5 Other methods for relieving chronic pain include radio waves, spinal cord stimulation, and nerve blocks.6 Many of these and other non-pharmacological options can be used along with prescribed medications; however it is best that the patient consults with their physician on the safety and effectiveness of these alternatives.7
Pharmacologic analgesia
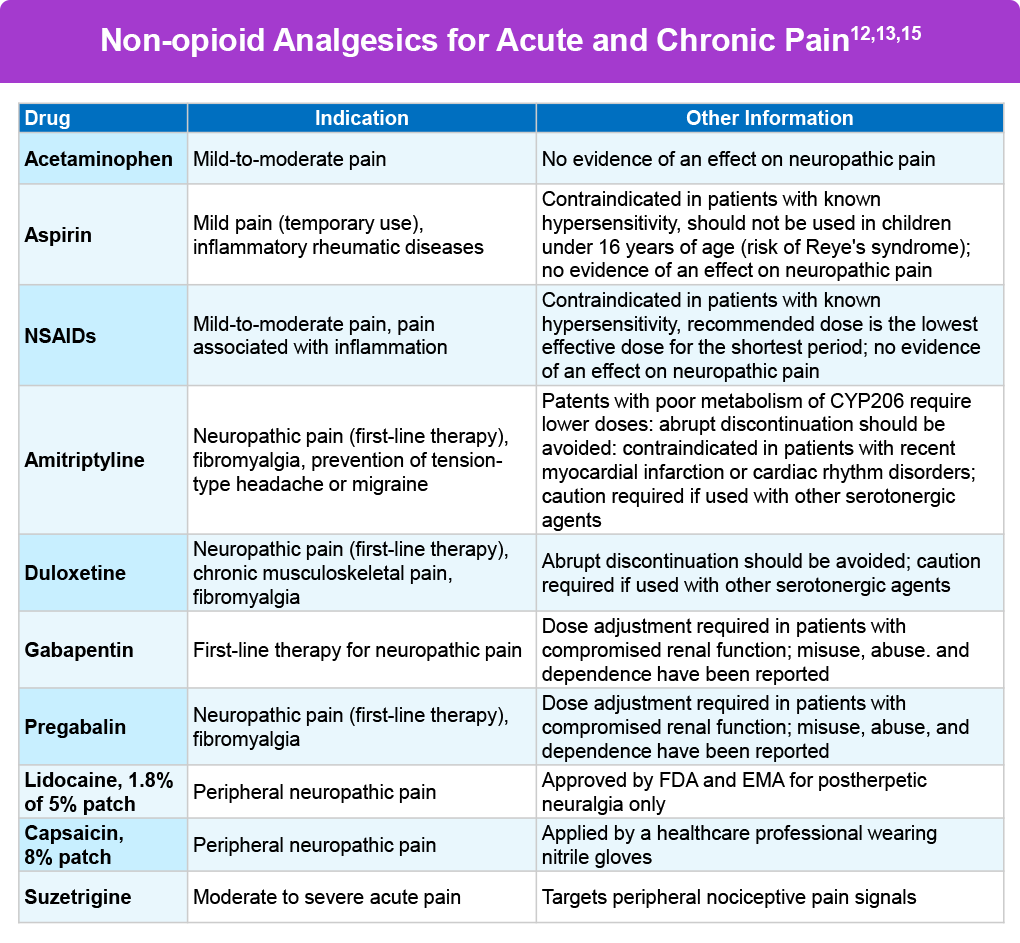
Pharmacologic analgesics are of two kinds: non-opioids and opioids. Non-opioids can include acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDS), such as aspirin and ibuprofen.6 Gabapentin can be prescribed for neuropathic pain and the anxiety people may have along with their chronic pain.7 Antidepressants, such as duloxetine and others can also be prescribed for neuropathic pain.7 Other non-opioid options include muscle relaxants, local anesthetics, ketamine, clonidine, and nitrous-oxide gas.9-11
In January 2025 the US Food & Drug Administration approved a first-in-class non-opioid medication for moderate to severe acute pain, called suzetrigine. Suzetrigine, the first such agent approved in 20 years, works by targeting the voltage-gaited sodium channel NAv1.8 in the peripheral nervous system.12 The novel mechanism of action circumvents the central nervous system, and therefore negates the addictive properties associated with opioid medications. The most common adverse reactions in clinical trial participants receiving suzetrigine were itching, muscle spasms, increased blood level of creatine phosphokinase, and rash.12,14
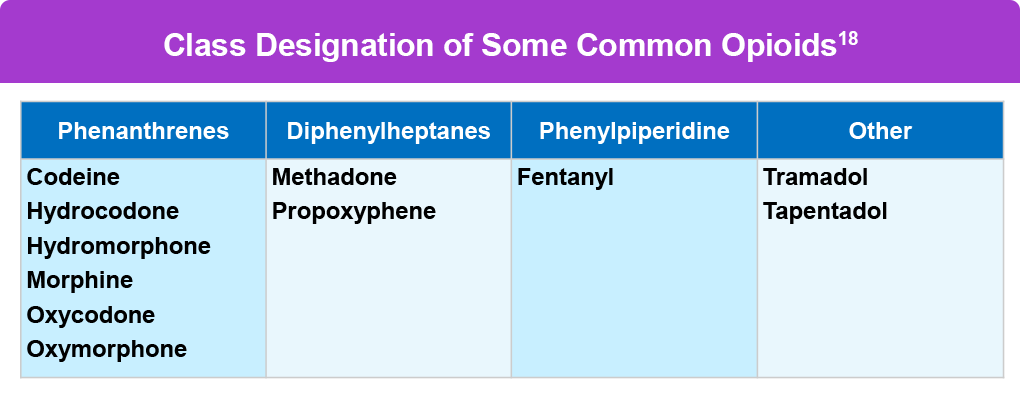
Opioids are powerful pain medications usually prescribed only when pain doesn’t respond adequately to other analgesics. Opioids may be naturally derived from the opium poppy plant, such as morphine, or synthetic and semi-synthetic and created in a laboratory, such as methadone and oxycodone. Common prescription opioids include hydrocodone, oxycodone, oxymorphone, morphine, codeine, and pharmaceutically made fentanyl.16 Opioids bind to opioid receptors in the brain and spinal cord and block pain signals, and also release dopamine into the body.16 It’s important to note pharmaceutical fentanyl is not the same as illegally manufactured or illicit fentanyl, which contributes to a majority of opioid overdoses and can also be mixed with other dangerous compounds.17
Opioids can be prescribed for both acute and chronic pain, and after surgeries, injuries, or cancer. Opioids for acute pain are used for only a few hours, or up to 3 days, and less commonly up to 5 or 7 days.19 Opioids are also grouped as short-acting, which stay in the bloodstream for a short while, or long-acting, which can be extended-release (ER) or sustained-release (SR), and are prescribed for people with chronic pain.20 Although the rate of opioid prescriptions has been declining, opioids are still a commonly prescribed class of pain medications. In 2022 alone there were more than 130 million opioid prescriptions in the US.5
Opioids as a Controlled Substance
There are many benefits of opioids for pain management, but they carry serious risks of misuse, addiction, overdose, and death.22 Due to these risks, opioids are considered a controlled substance per the Controlled Substances Act of the US Drug Enforcement Administration (DEA), where regulated drugs are placed into five different categories or schedules.23 Scheduling is determined by a drug’s medical use and potential for misuse, as well as scientific evidence, public health risks, dependence liability, pharmacological effect, and other factors.23 Schedule I Controlled Substances have no currently accepted medical use in the US, and they have a high potential for abuse (eg, heroin).23 Schedule V drugs have the lowest potential of abuse relative to the other categories (eg, limited quantities of narcotics in cough syrups).23 Most opioids fall into schedules II-IV, and most opioids for chronic pain treatment in Schedule II. Each state has different regulations on the dosage and supply limitations for opioids, and physicians must consult local laws prior to prescribing any opioid.24
References
- Haefeli M, Elfering A. Pain assessment. Eur Spine J. 2006;15(suppl 1):S17-S24.
- Staats P, et al. Understanding the role of patient preference in the treatment algorithm for chronic low back pain: Results from a survey-based study. Pain Manag. 2022;12:371-382.
- National Center for Complementary and Integrative Health. Chronic pain: What you need to know. Last updated January 2023. https://www.nccih.nih.gov/health/chronic-pain-what-you-need-to-know
- Licciardone JC, et al. Osteopathic manipulation in the management of chronic pain: Current perspectives. J Pain Res. 2020;13:1839-1847.
- Centers for Disease Control and Prevention (CDC). Nonopioid therapies for pain management. May 2, 2024. https://www.cdc.gov/overdose-prevention/hcp/clinical-care/nonopioid-therapies-for-pain-management.html
- American Society of Anesthesiologists (ASA). Non-Opioid treatment for chronic pain. 2024. https://www.asahq.org/madeforthismoment/pain-management/non-opioid-treatment/
- Dey S, et al. Alternatives to opioids for managing pain. StatPearls. Last updated April 21, 2024. https://www.ncbi.nlm.nih.gov/books/NBK574543/
- Castellano-Tejedor C. Non-pharmacological interventions for the management of chronic health conditions and non-communicable diseases. Int J Environ Res Public Health. 2022;19:8536.
- Bell RF, Kalso EA. Ketamine for pain management. Pain Rep. 2018;3:e674.
- Duma A, et al. The effect of nitrous oxide anesthesia on early postoperative opioid consumption and pain. Reg Anesth Pain Med. 2014;39:31-36.
- Helander EM, et al. Multimodal analgesia, current concepts, and acute pain considerations. Curr Pain Headache Rep. 2017;21:3.
- U.S. Food & Drug Administration. FDA Approves Novel Non-Opioid Treatment for Moderate to Severe Acute Pain. January 30, 2025. https://www.fda.gov/news-events/press-announcements/fda-approves-novel-non-opioid-treatment-moderate-severe-acute-pain
- Jones J, et al. Selective Inhibition of NaV1.8 with VX-548 for Acute Pain. N Engl J Med. 2023 Aug 3;389(5):393-405.Selective Inhibition of NaV1.8 with VX-548 for Acute Pain. N Engl J Med. 2023 Aug 3;389(5):393-405.
- ABC News. FDA approves new type of non-opioid pain medication, 1st of its kind in more than 20 years. January 30, 2025. https://abcnews.go.com/Health/fda-approves-new-type-opioid-pain-medication-1st/story?id=118266291
- Finnerup NB. Nonnarcotic methods of pain management. N Engl J Med. 2019;380:2440-2448.
- National Institute on Drug Abuse (NIDA). Prescription Opioids Drug Facts. https://nida.nih.gov/publications/drugfacts/prescription-opioids
- Centers for Disease Control and Prevention (CDC). Fentanyl Facts. April 2, 2024. https://www.cdc.gov/stop-overdose/caring/fentanyl-facts.html
- Veterans’ Health Administration & Defense Health Agency. Use of Opioids in the Management of Chronic Pain Work Group: VA/DoD Clinical Practice Guideline. May 2022. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
- Dowell D, et al. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. JAMA. 2016;315:1624–1645.
- US Food and Drug Administration (FDA). FDA Education Blueprint for Health Care Providers Involved in the Treatment and Monitoring of Patients with Pain. October 2023. https://www.fda.gov/media/173774/download?attachment
- Rach M. Which opioids are prescribed today and when are they used? Mayo Clinic Press. Published January 17, 2023. https://mcpress.mayoclinic.org/opioids/types-of-opioids/
- US Food and Drug Administration (FDA). All Opioid Pain Medicines: Drug Safety Communication. April 2023. https://www.fda.gov/safety/medical-product-safety-information/all-opioid-pain-medicines-drug-safety-communication-fda-updates-prescribing-information-provide
- Drug Enforcement Administration (DEA). Drugs of Abuse. April 13, 2020. https://www.dea.gov/documents/2020/2020-04/2020-04-13/drugs-abuse
- Stone EM, et al. Association between state opioid prescribing cap laws and receipt of opioid prescriptions among children and adolescents. JAMA Health Forum. 2022;3:e222461.
All URLs accessed September 11, 2025.